Journal: bioRxiv
Article Title: CD33 and clusterin interact biophysically and genetically to modulate Alzheimer risk
doi: 10.1101/2025.07.29.667318
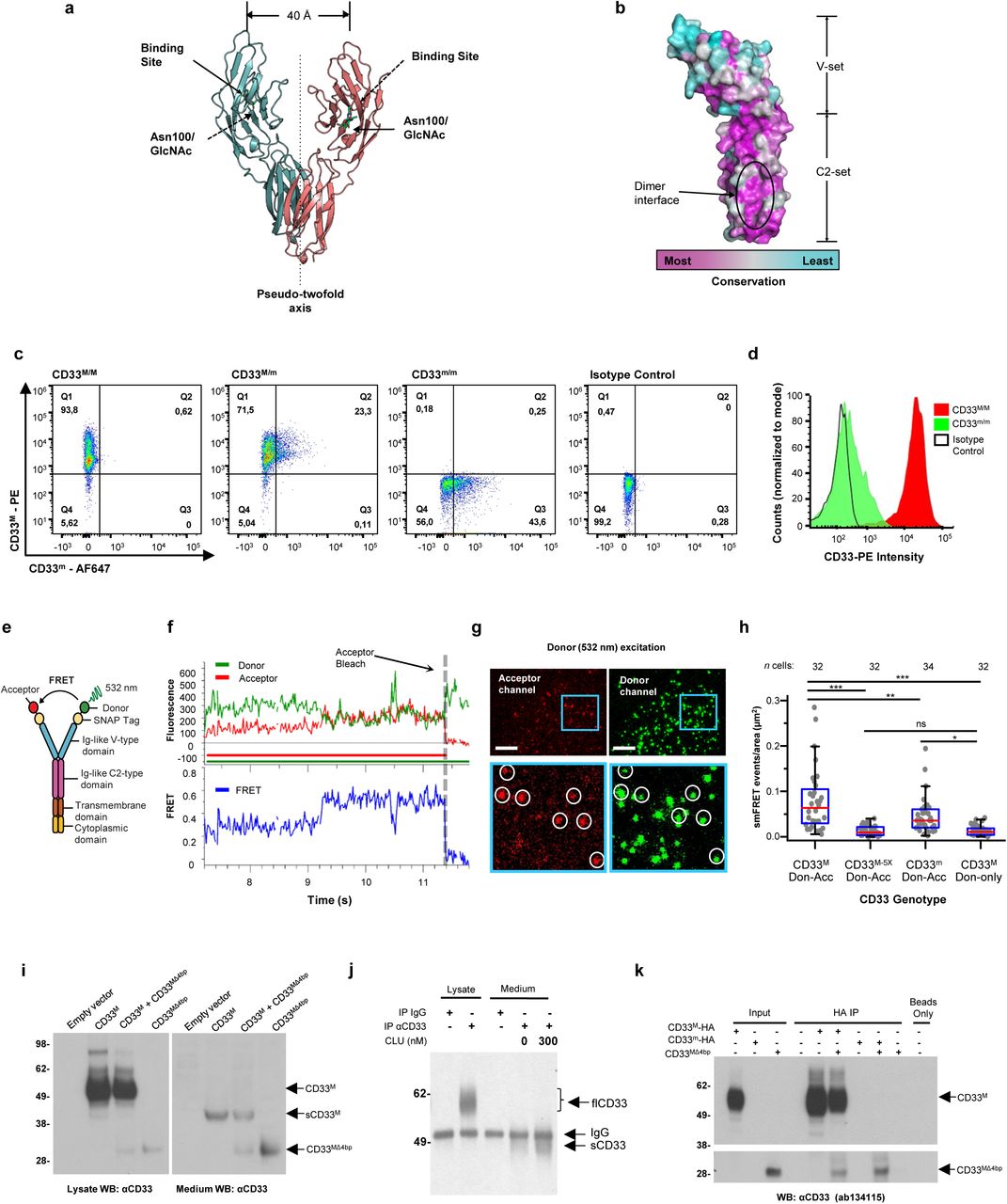
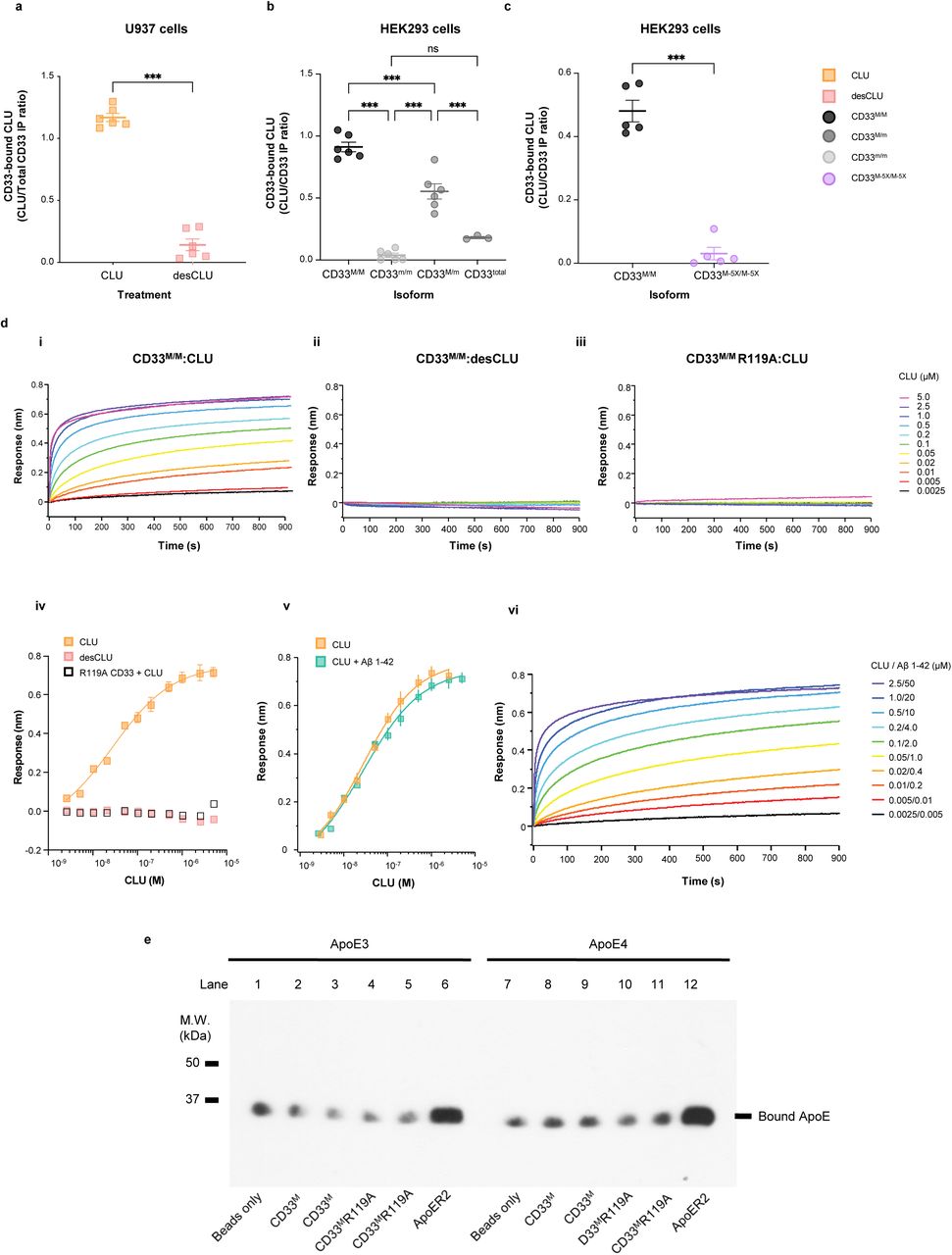
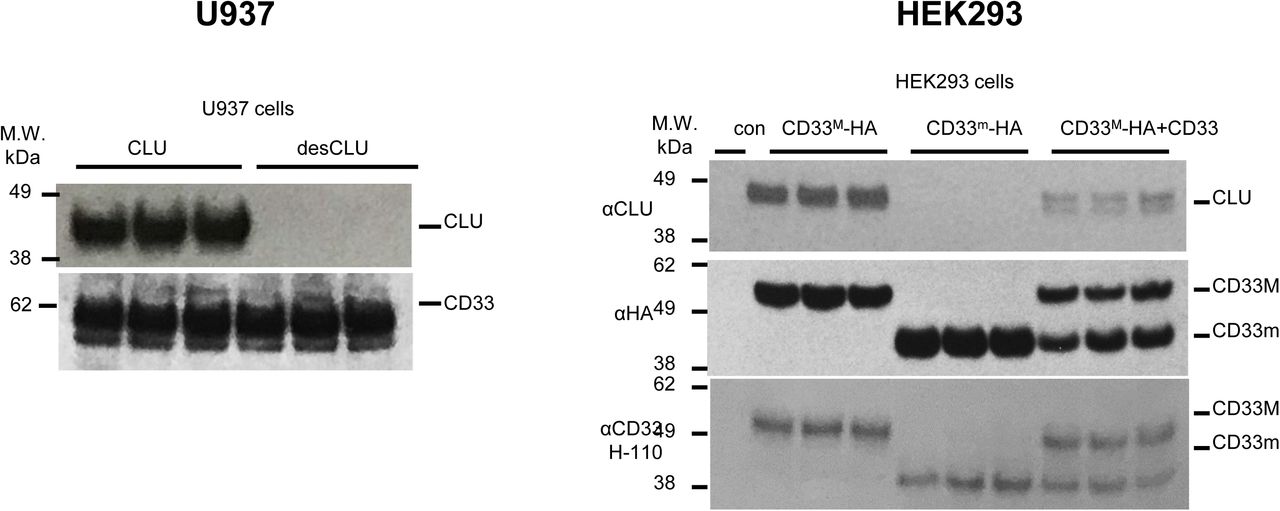
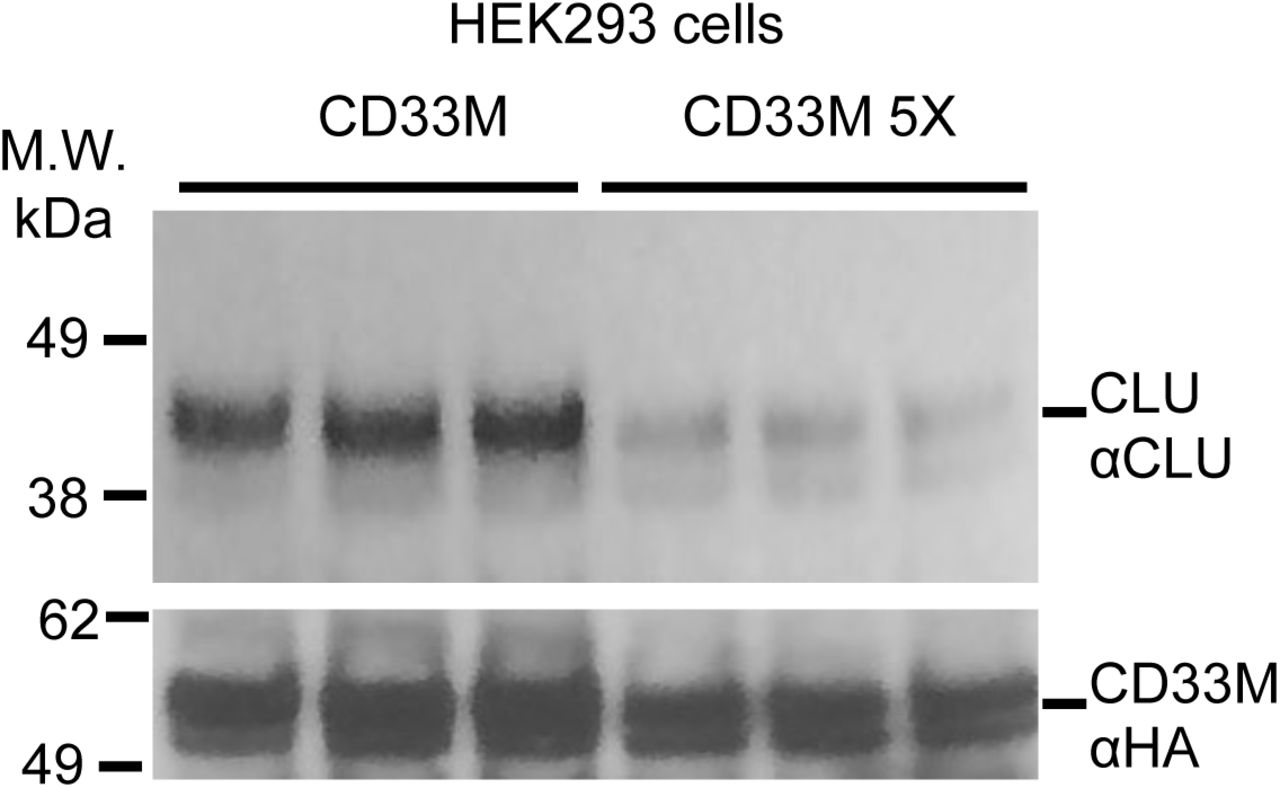
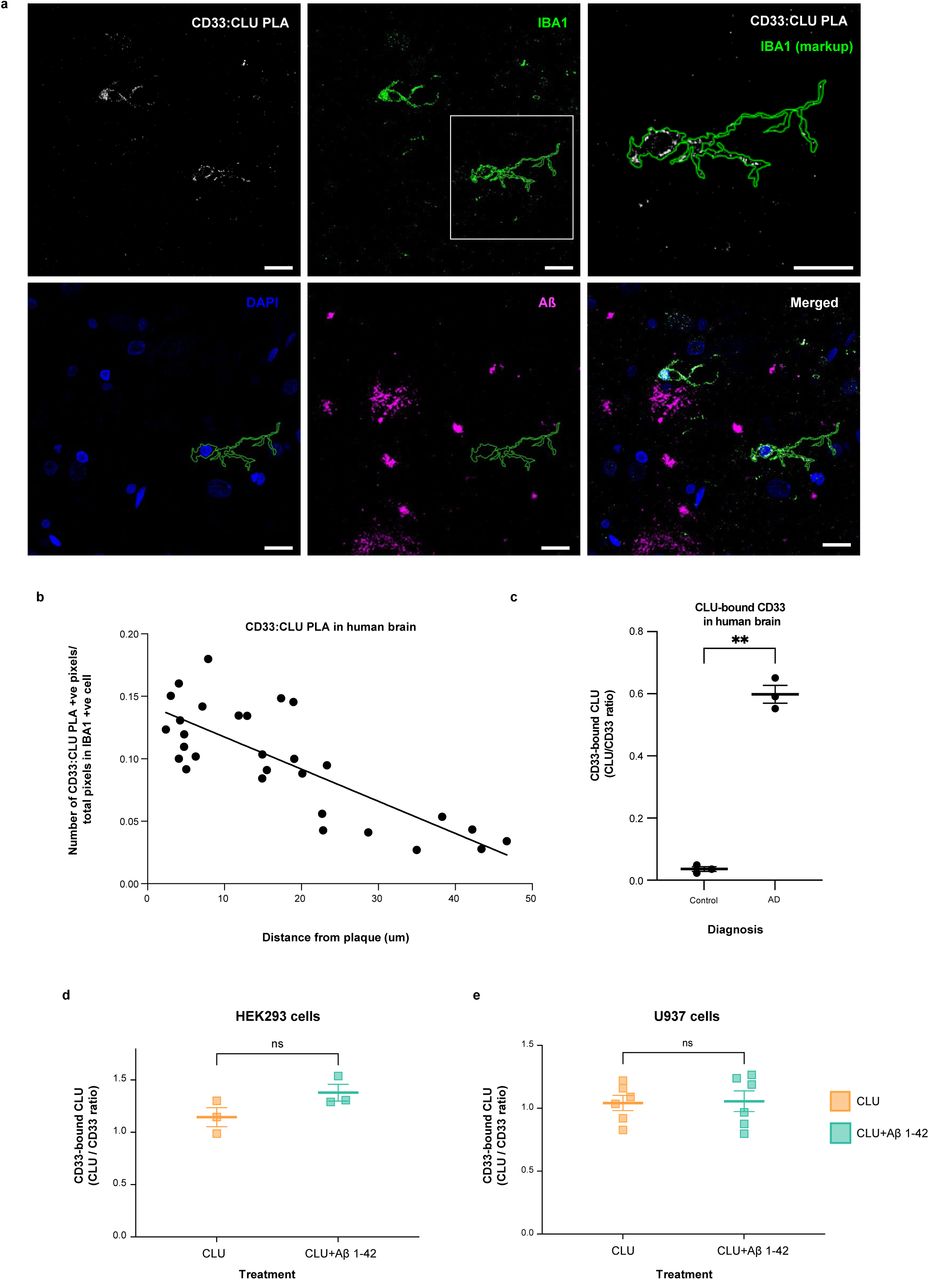
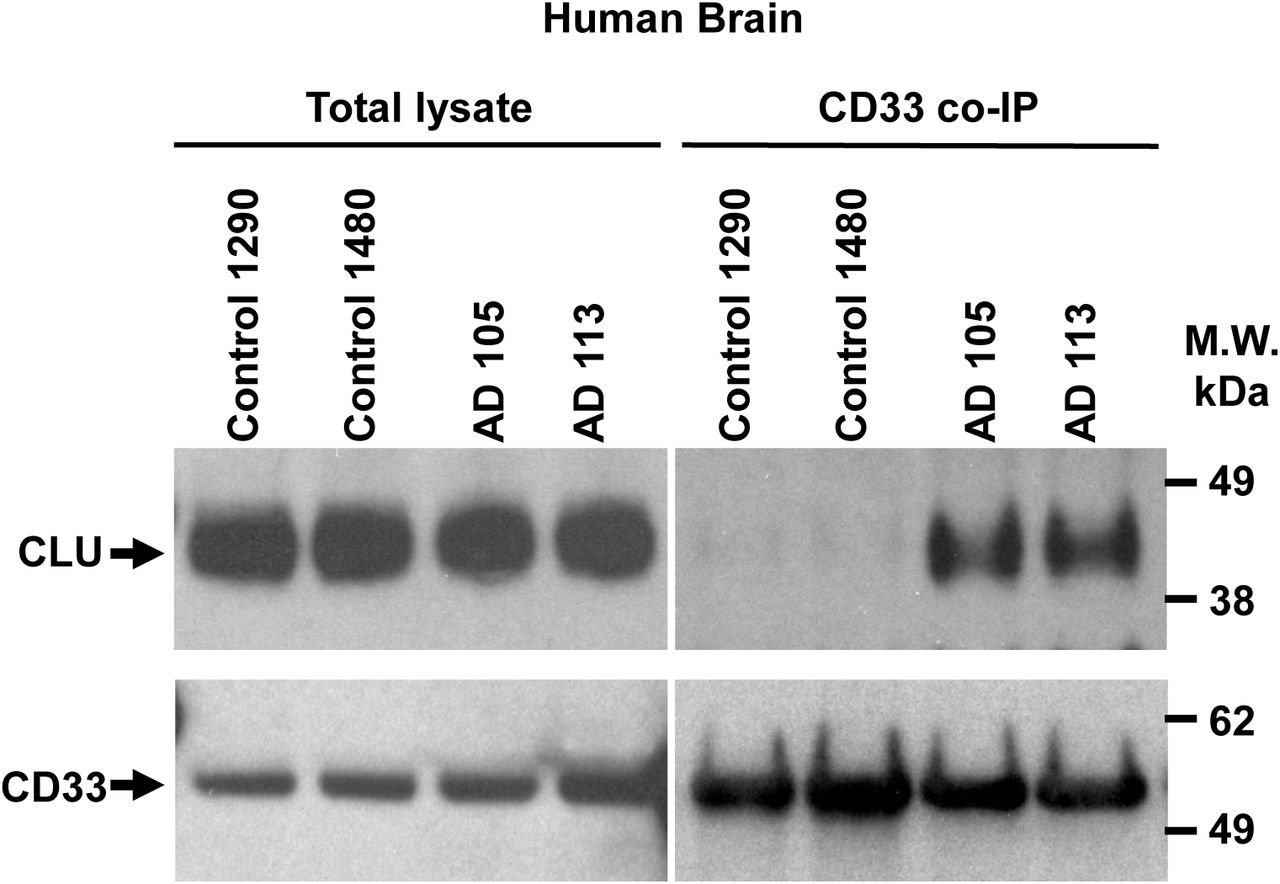
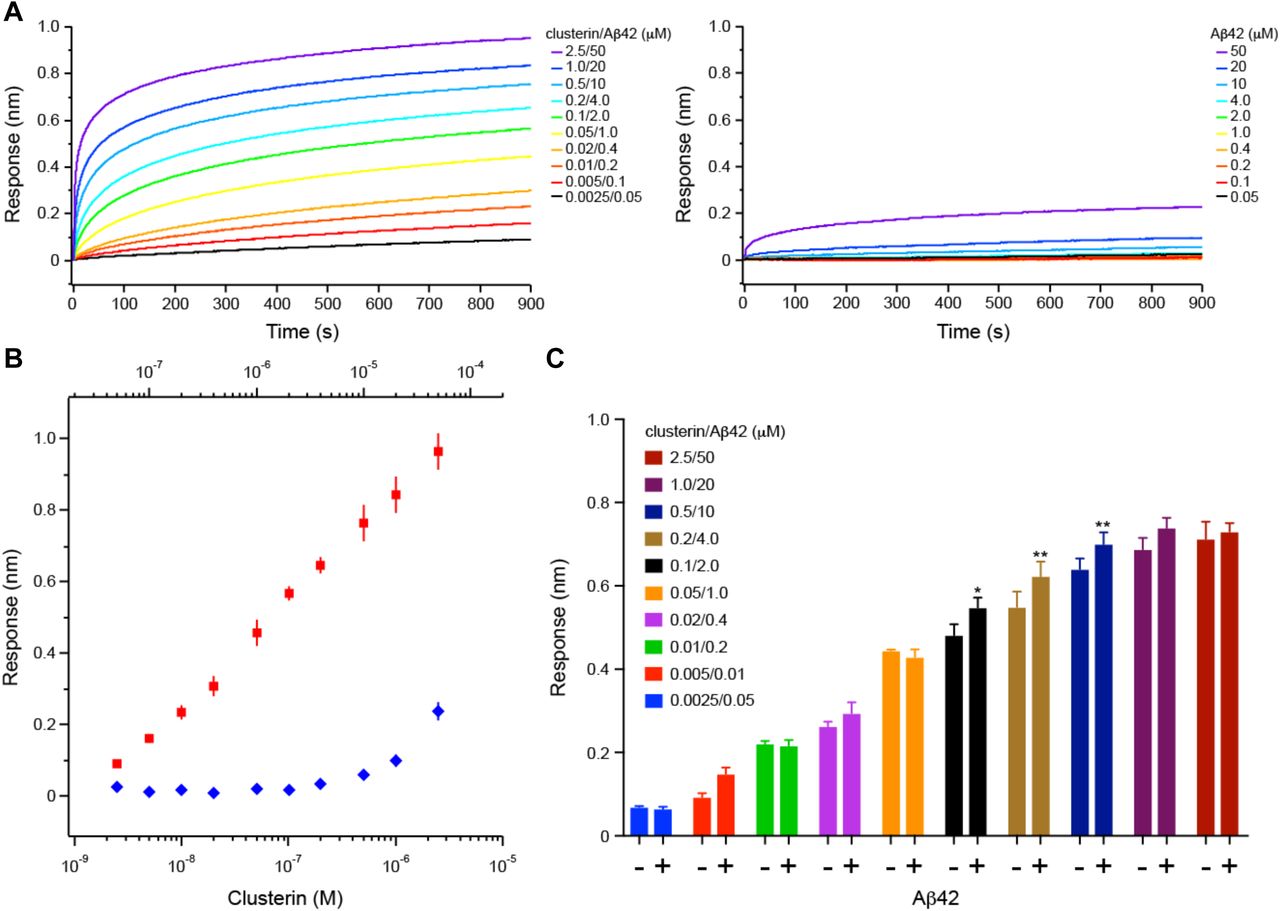
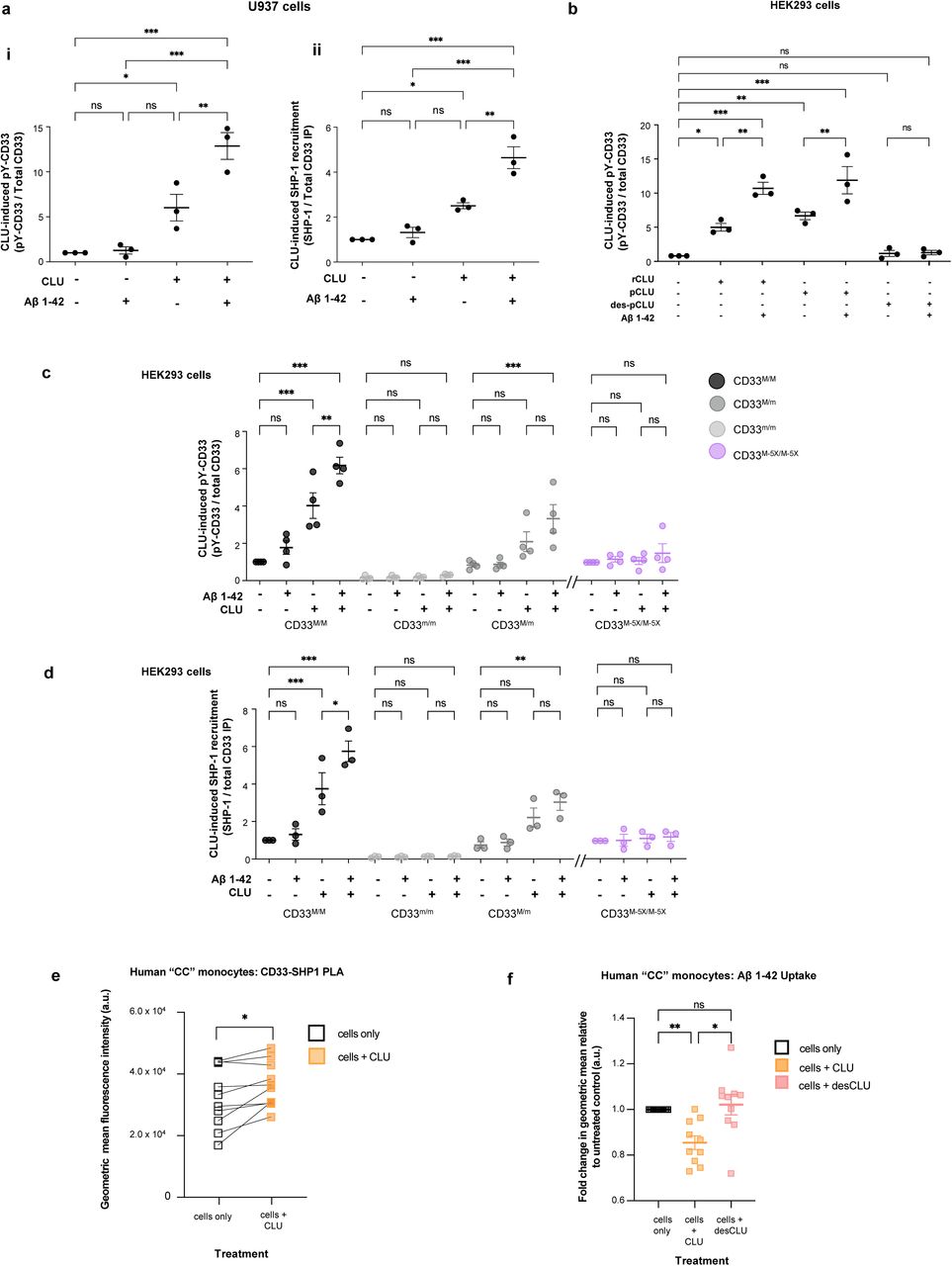
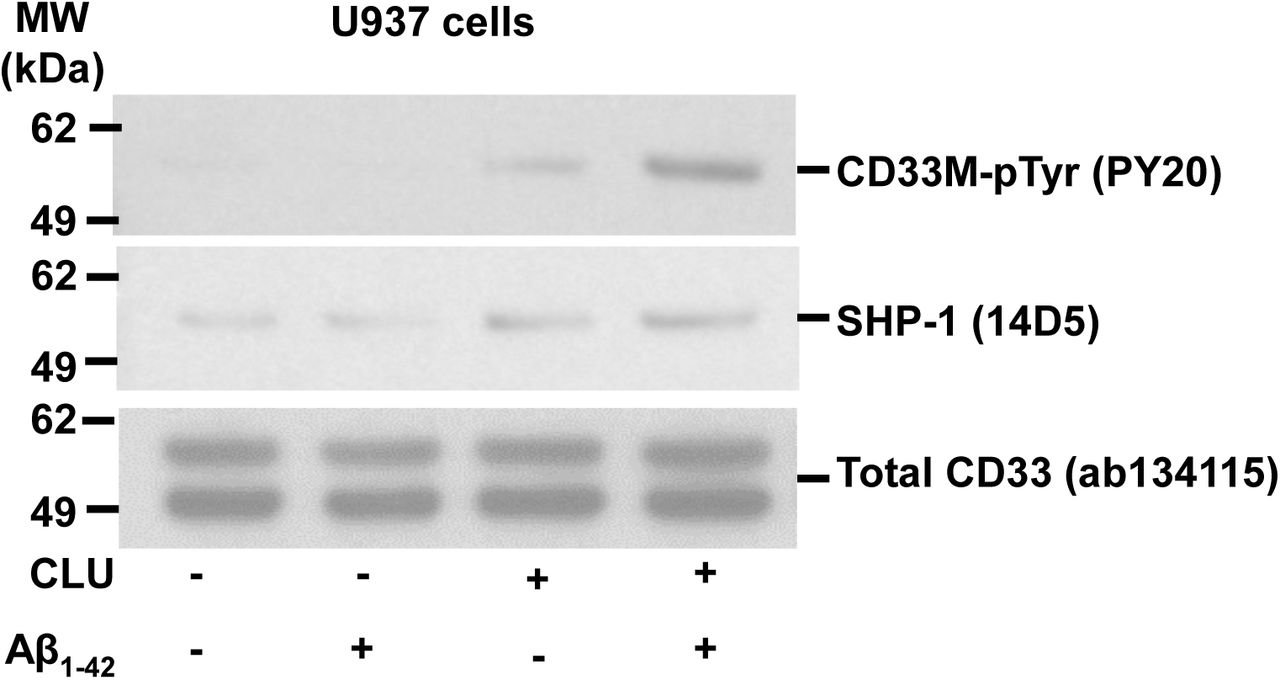
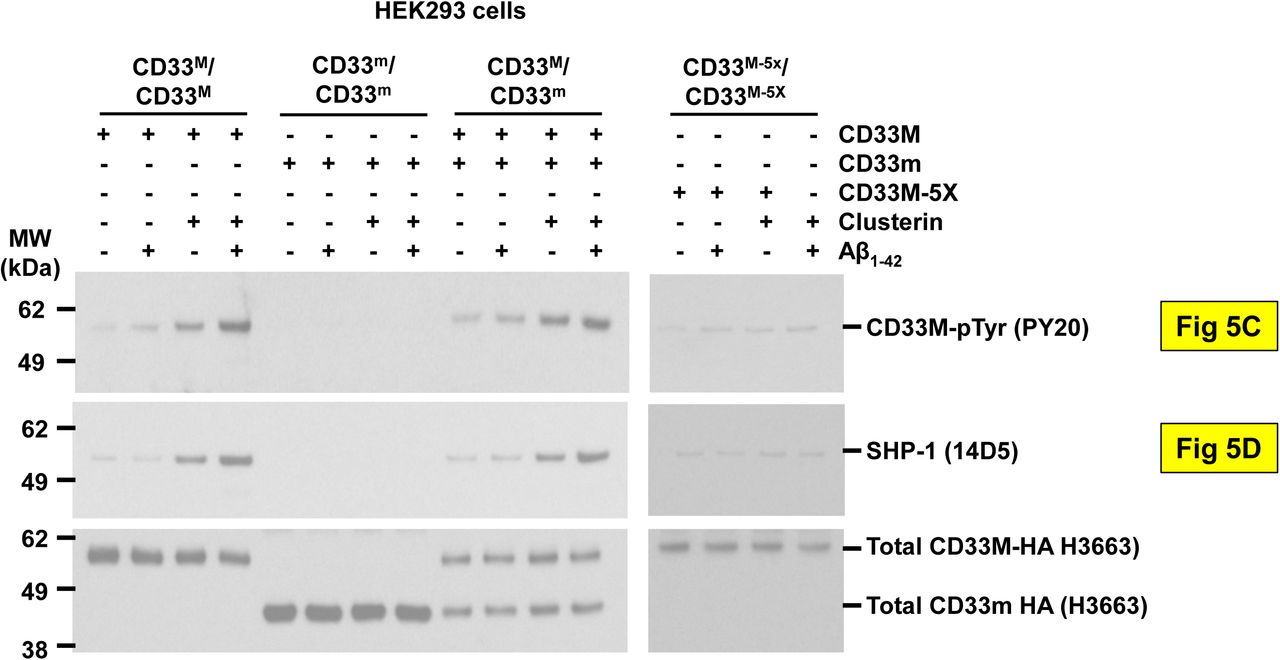
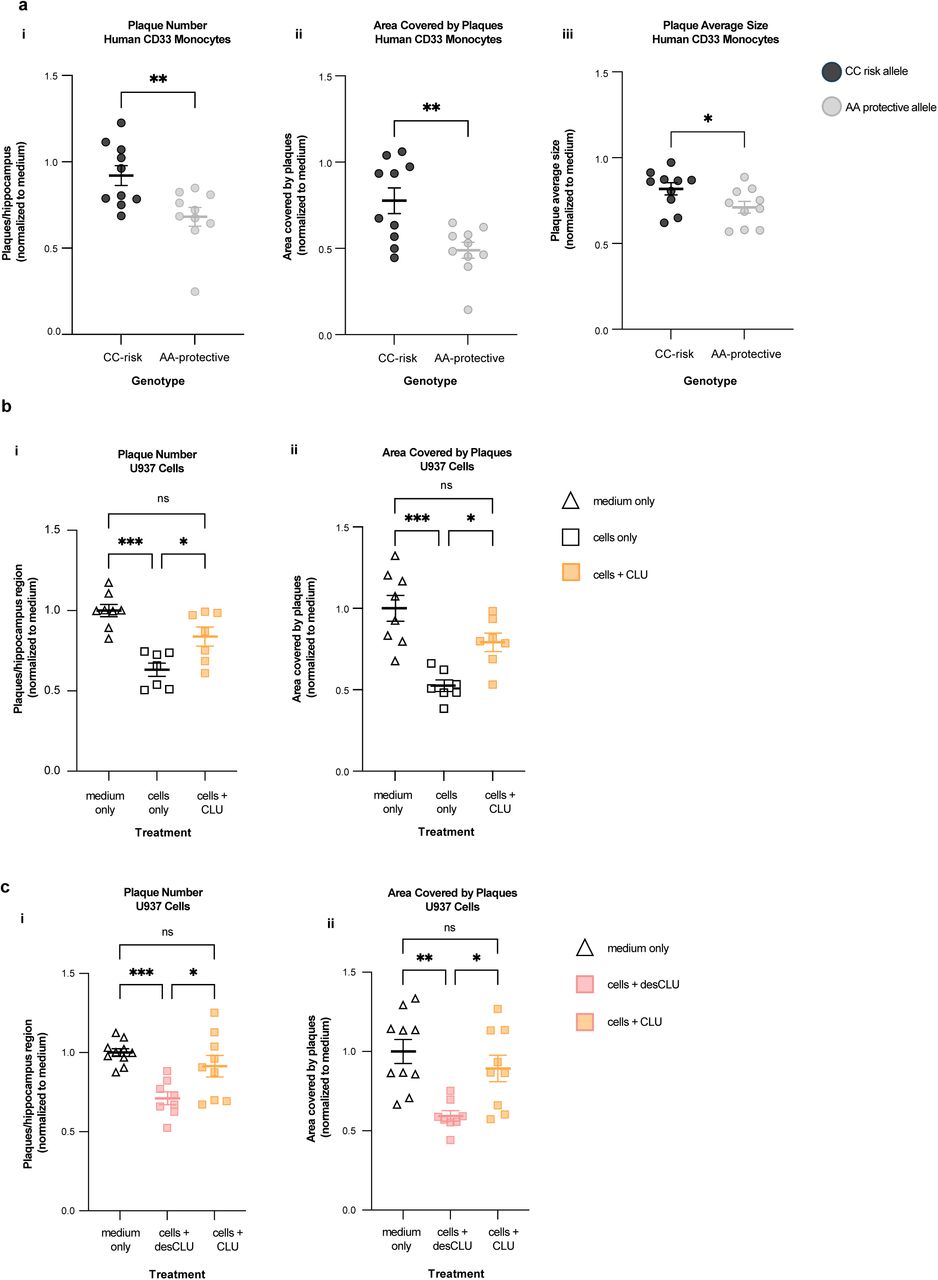
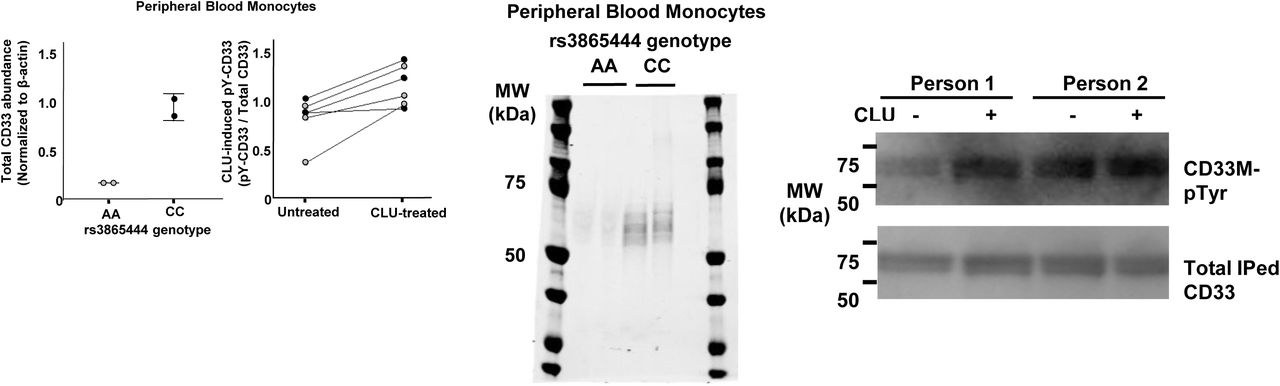
Figure Lengend Snippet: a. Cartoon showing the dimerization of two CD33 M ectodomain chains (teal and pink) mediated by their C2-set domains. The two C strands hydrogen bond in a parallel fashion to create a continuous β-sheet (GFCCFG) and the remainder of the interface is formed by residues from the CD33-specific D strand. The C2-set dimeric interface and the rigid nature of the V-set/C2-set interface places the two ligand binding sites 40 Å apart, facing outwards from the central axis of the dimer and pointing away from one another. b. Surface representation of CD33 M ectodomain colour-coded according to conservation (purple – conserved, cyan – non-conserved). The conserved patch constituting the dimer interface is indicated. c. Dimeric CD33 M is robustly targeted to the cell surface in both CD33 M /CD33 M and CD33 M /CD33 m expressing cells : representative flow cytometry analyses showing CD33 M and CD33 m surface labelling in cells expressing CD33 M only (CD33 M / M ), CD33 m only (CD33 m / m ) or CD33 M and CD33 m (CD33 M / m ), reveals that the majority of both CD33 M / M and CD33 M / m cells have similarly robust surface exposure of CD33 M . We were not able to include CD33 M-5X cells in these experiments because the 5X dimer site mutant disrupts the recognition motif for the anti-CD33 antibody that recognizes CD33 M in fluorescence flow cytometry. Cells were double stained using antibodies specific to CD33 M (WM53) and CD33 m (A16121H) respectively shows, in the displayed experiment (see ), that most CD33 M / M cells (upper left, Q1+Q2: 93.8% + 0.62%) or CD33 M / m cells (upper right, Q1+Q2: 71.5% + 23.3%) are surface CD33 M positive. Only a small portion of cells in CD33 M / m cells (upper right, Q2+Q3: 23.3% + 0.11%) are CD33 m positive. In the CD33 m / m (lower left, Q2+Q3: 0.25% + 43.6%) cells, CD33 m positive cells are less than a half of the total cells. Figures show one experiment out of three independent experiments (each with 6 technical replicates) with similar results. The MFI statistics of CD33M and CD33m from three independent experiments are shown in Extended Figure 2J d. Single staining using the HIM3-4 antibody, which recognises both CD33 M and CD33 m , shows much greater surface exposure of CD33 M in CD33 M / M cells (red) than there is surface exposure of CD33 m in CD33 m / m (green) cells. e. Schematic of single-molecule TIRF imaging of CHO cells expressing SNAP f -CD33 M labeled with donor and acceptor fluorophores . The colours on the dimer cartoon reflect the same CD33 domains as in . f. Representative smFRET trajectory and its corresponding fluorescence and FRET time traces for the CD33 M receptor (bottom panel). The smFRET trajectory for the individual molecule is shown to the left of its fluorescence (donor and acceptor trajectories and intensities are shown in green and red, respectively) and FRET trace (in blue). The green and red bars along the time axis in the fluorescence time trace plot indicate that the signal was derived during tracking. g. Top panels: Representative image from a movie (frame 2, 0.03 s) of labeled SNAP f - CD33 M e xcited by the donor laser (532 nm), with the enlarged view (bottom panels) showing sensitized acceptor signals that have colocalizing trajectories with their corresponding donors, both delineated by white circles. (See cartoon in Supplementary Figure 2K for methodology) Scale bar, 5 μm; enlarged view, 9.1 μm × 7.3 μm. Donors without colocalized acceptor are consistent with the stochastic labeling approach employed in which a population of dimers labeled with two donors or only one donor and no acceptor is possible. h. Distributions of smFRET events per cell area for SNAP f -CD33 M , -CD33 M-5X , and -CD33 m , all labeled with donor and acceptor (Don-Acc) as well as SNAP f -CD33 M labeled with only donor (Don-only). The smFRET events represent the total number of freely diffusing smFRET trajectories (see cartoon in Supplementary Figure 2K for methodology). Dots represent smFRET events per area for each cell. Box plots indicate the median (value shown as the central line) and interquartile range (lower and upper lines represent the 25th and 75th percentiles, respectively), while the whiskers represent the points that fall within 1.5 × interquartile range. The indicated total number of cells ( n cells) were collected over 4 independent experiments for the CD33 M and CD33 m samples, and 3 independent experiments for the CD33 M-5X and CD33 M donor only samples. ***, p = 1.3 × 10 -8 ; ***, p = 1.6 × 10 -8 ; **, p = 0.0056; *, p = 0.011; ns = 0.99; ordinary one-way ANOVA with Šídák’s post- hoc multiple comparisons test. i. CD33 MΔ4bp variant forms a stable soluble protein in cell lysates and is secreted into the media , where it may bind with the extracellular domains of CD33 M and CD33 m . There may be a similar physiological soluble CD33 protein that is either shed into the medium by CD33 M ectodomain proteolysis or is produced as an alternative splicing product. A cartoon showing the domain structures and epitope tags of CD33 M , CD33 m , CD33 Δ4bp and the putative soluble CD33 M ectodomain product (sCD33 M ) is included in . j. Western blot analysis of CD33 immunoprecipitated by the CD33 Ig-like V-set domain antibody (ab134115) from lysates and conditioned medium of iMGs . Immunoprecipitation products were probed for CD33 using the CD33 Ig-like C2-set domain antibody (PWS44). The data demonstrate the presence of fuzzy, likely glycosylated soluble CD33- immunoreactvive (sCD33) cleavage product in the medium, but not in iMG cell lysates. Notably, sCD33 increases upon stimulation of the iMG cells with 300 nM CLU (lane 5). k. Western blot of lysates and HA-immunoprecipitation products from HEK293 cells expressing CD33 M -HA, CD33 m -HA and CD33 MΔ4bp. HA-immunoprecipitation products were probed with the anti-CD33 antibody (ab134115) which recognises only epitopes in the V-set domain. Arrowheads denote CD33 derivative protein. Representative blots for n = 3 independent biological replicates. The result reveals that CD33 MΔ4bp co-precipitates with CD33 M and CD33 m . These studies do not distinguish whether this interaction occurs in the intracellular compartment or whether they occur at the cell surface.
Article Snippet: In the first antibody incubation, rabbit anti-human CD33 (Bioss, bs-2042R, 1:400) and mouse anti-human SHP-1 (Thermo Scientific, MA5-38614, 1: 400), were performed overnight at 4°C.
Techniques: Ligand Binding Assay, Expressing, Flow Cytometry, Mutagenesis, Fluorescence, Staining, Imaging, Labeling, Derivative Assay, Variant Assay, Produced, Alternative Splicing, Western Blot, Immunoprecipitation